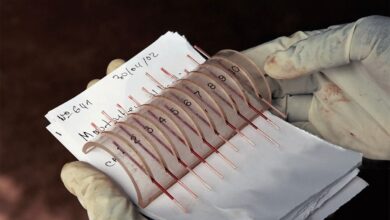
Kendric Cromer, 12, the first commercial patient for Bluebird Bio’s gene therapy to cure his sickle cell disease, in the hospital as his bone marrow stem cells were being removed for gene editing. Photo Credit: Kenny Holston
Health USASickle Cell Disease: First Patient to Receive Treatment
Kendric Cromer, a 12-year-old boy from Washington, the United States, is the world’s first patient who has sickle cell disease to undergo a commercially approved gene therapy, one that gives hope to thousands of people dealing with the painful genetic disorder of red blood cells.
“We always prayed this day would come,” says Deborah, Kendric’s mom. “I want to play basketball,” adds Kendric who wants to become a geneticist when he grows up.
At the end of 2023, the Food and Drug Administration authorized two companies – Bluebird Bio and Vertex Pharmaceuticals – to sell their gene therapy. Some 20,000 Americans who have sickle cell disease qualify for the treatment. The process is so time-consuming that Bluebird estimates it can treat between 85 and 105 patients annually. The monthslong therapy consists of removing the bone marrow stem cells, which are then genetically modified in a specialized lab for the treatment. Once the patient’s stem cells have been treated, they stay in the hospital for a month under expert and intensive care as they become severely ill from potent chemotherapy. Bluebird’s gene therapy Lyfgenia costs $3.1 million. It is one of the highest prices ever for a treatment, and some insurance companies agree to cover it.